Read the latest blogs from across our work within the secondary breast cancer community. From blogs on fundraising, research findings, information sharing, corporate achievements, community support and stories, celebrate with us as we work together to Make 2nds Count.

Vicki's story
Exploring clinical trials can be an important part of your treatment journey. Our Patient Trials Advocate nurse Mel can help answer any questions that you might have and do a clinical trial database search for you.

2023 Education Round Up
This year clinical trials was a key area of focus for our Education programmes, helping people living with secondary (metastatic) breast cancer to access the information they need to explore the option of clinical trials as part of their treatment pathway. Our Patient Trials Advocate service has really taken off this year!

Review of Clinical Trials in the UK
An independent review, commissioned by the UK government and led by Lord O’Shaughnessy, has recently been published with many recommendations on how to tackle the key challenges in delivering commercial clinical trials and how the environment for commercial clinical trials can be improved in the UK. Although the UK has an illustrious track record in the area of clinical trials, both historically and with recent successes, such as with the COVID-19 vaccine and therapeutic COVID drug trials, in recent years the UK has started to fall behind in commercial clinical trial activity.

New Actively Recruiting Clinical Trials in the UK for Spring 2023
Every month Make 2nds Count updates its registry of all actively recruiting secondary breast cancer clinical trials in the UK. This post highlights some of the new clinical trials added to our registry that are actively recruiting participants within the UK for Spring 2023.

UK Clinical Trial Registry
We are proud to launching our Clinical Trial Registry to expand on our Patient Trial Advocate service. Our esteemed chairperson, Proffessor David Cameron, comments on the project: “We realise that many patients with secondary breast cancer want, and deserve, the chance to consider taking part in a clinical trial.

A research highlight from ESMO 2022 - the TROPiCS-02 study
What is ESMO?The European Society for Medical Oncology (ESMO) is a leading professional organisation with more than 25,000 members from over 160 countries. The 2022 ESMO congress took place in Paris, France in a hybrid manner between the 9th - 13th September with an estimated 29,300 attendees.

Clinical Trials Day
When I was diagnosed out of the blue in March 2014 with advanced breast cancer, I never thought that I would be living with a good quality of life 8 years on. I’m Her2 positive, and spent my first 18 months post diagnosis rattling through all the standard treatments, (including an expensive self-funded spell on Kadcyla before it was approved in Scotland – and which didn’t work,).

Life on a Phase 1 clinical trial
When I was told in late 2015 that I’d secured the last place on a phase 1 clinical trial, I didn’t in my wildest dreams think I’d end up still on that trial almost four years later. I had been diagnosed de novo (another unnecessarily complicated medical term which means ‘from the start’) in 2014 with Her2 breast cancer that had already spread to my liver, lungs and bones – then a year later into my brain.
Trials Experience: Joanna
When were you diagnosed?I was told over the phone by my GP on Friday 13th Oct 2017 that it looked like my cancer was back after I asked for some tests on rib pain. I had no idea this could be a possibility!

Trial Experiences: Kate
When were you diagnosed?Feb 28th 2019 - was diagnosed with stage 3 ER+ breast cancer. March 13th 2019 - was told it had spread to my bones and possibly liver.
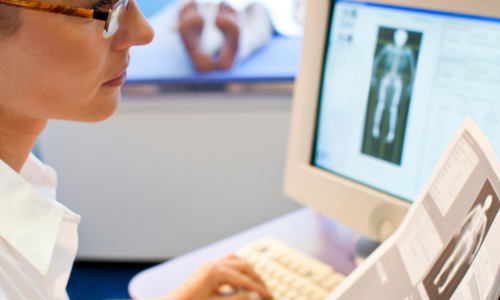
Patient Testimony: Bone Density Scan
Bone Density Scans or Dual Energy X-Ray Absorptiometry (DEXA) scans, use a low dose of X-ray to establish the density (or strength) of bones. Bone density scans are used to diagnose or assess the risk of osteoporosis, a condition that makes bones weak and likely to break (fracture).

Trial Experiences: Pamela
When were you diagnosed ? My original diagnosis was in July 2012 after a routine mammogram.

The essential restart of clinical trials to give secondary breast cancer patients hope
It has been widely reported in the last week that in the UK recruitment to clinical trials for cancer including secondary breast cancer have been stopped because of Covid-19. As a result, many stage 4 patients are missing out on potentially life-saving access to drugs that could keep them alive for longer.
The Impact of Covid-19 On Trials & Medical Research
The Impact of Covid-19 On Trials & Medical ResearchLast month, in the UK the NIHR Clinical Research Network announced it is pausing the site set up of any new or ongoing studies at NHS and social care sites that are not nationally prioritised COVID-19 studies. While this is of course necessary to enable the research workforce to focus on combatting the pandemic or redeployment to frontline care, the implications for secondary breast cancer patients are huge.
Why are recruitment rates for trials dropping?
Why isn’t every patient with cancer offered a clinical trial? Why are my six little pills not available to every metastatic Her2 patient now?
